Info about the Vail Resorts Lawsuit
If you are visiting our website to inquire about the recent class action lawsuit against Vail Resorts, please click here for more information.
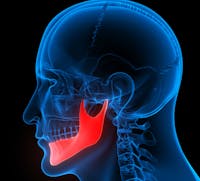
In the interest of helping patients with mandibular defects, the DePuy Synthes Craniomaxillofacial Distraction System is an implant designed to lengthen and/or stabilize the lower jawbone. It can be used on pediatric and adult patients alike to correct jaw-related defects that are either congenital or post-traumatic. It stabilizes the lower jaw by lengthening the bone, otherwise known as distraction.
In ideal working order, a craniomaxillofacial distraction system effectively lengthens the jaw using distraction osteogenesis. This means it slowly separates the bone, allowing new bone to grow naturally and fill in the space. However, the DePuy Synthesis Craniomaxillofacial Distraction System has shown a record of failure that puts patients at risk, prompting an FDA recall of the device.
The DePuy distraction system may reverse direction after surgery, reducing the distraction it was intended to produce. This failure puts infants at highest risk of injury, because the device could lead to rapid obstruction of the trachea. This may lead to respiratory collapse resulting in death.
Children or adults may be able to maintain airflow in the event of device failure. However, device failure may result in the need for surgical repair to replace the failed device.
As of April 2014, there were 15 reports of personal injury associated with the DePuy Synthes Craniomaxillofacial Distraction System.
At the law offices of Meyers & Flowers, we have the expertise and experience to pursue litigation against large medical firms such as DePuy. Our long record of excellence includes million-dollar awards on behalf of clients harmed by medical device failure.
If you received a DePuy distraction system and are seeking legal recourse, we can help. Your first step is a free consultation with our law professionals to discover if you have a case. It costs nothing to find out your options, and you pay no fees until we win your case. Please call us today to find out more.
© Meyers & Flowers Trial Attorneys. All Rights Reserved. Web Design & Internet Marketing by Studio III